
Separations on reversed-phase media are usually done in mixtures of aqueous and organic solvents, which are often denaturing conditions. Samples are loaded onto the matrix in a high-salt buffer and elution is by a descending salt gradient. Separations on HIC matrices are usually done in aqueous salt solutions, which generally are nondenaturing conditions. Hydrophobic interaction chromatography (HIC) and reversed-phase chromatography (RPC) are two separation methods based on the interactions between the hydrophobic moieties of a sample and an insoluble, immobilized hydrophobic group.
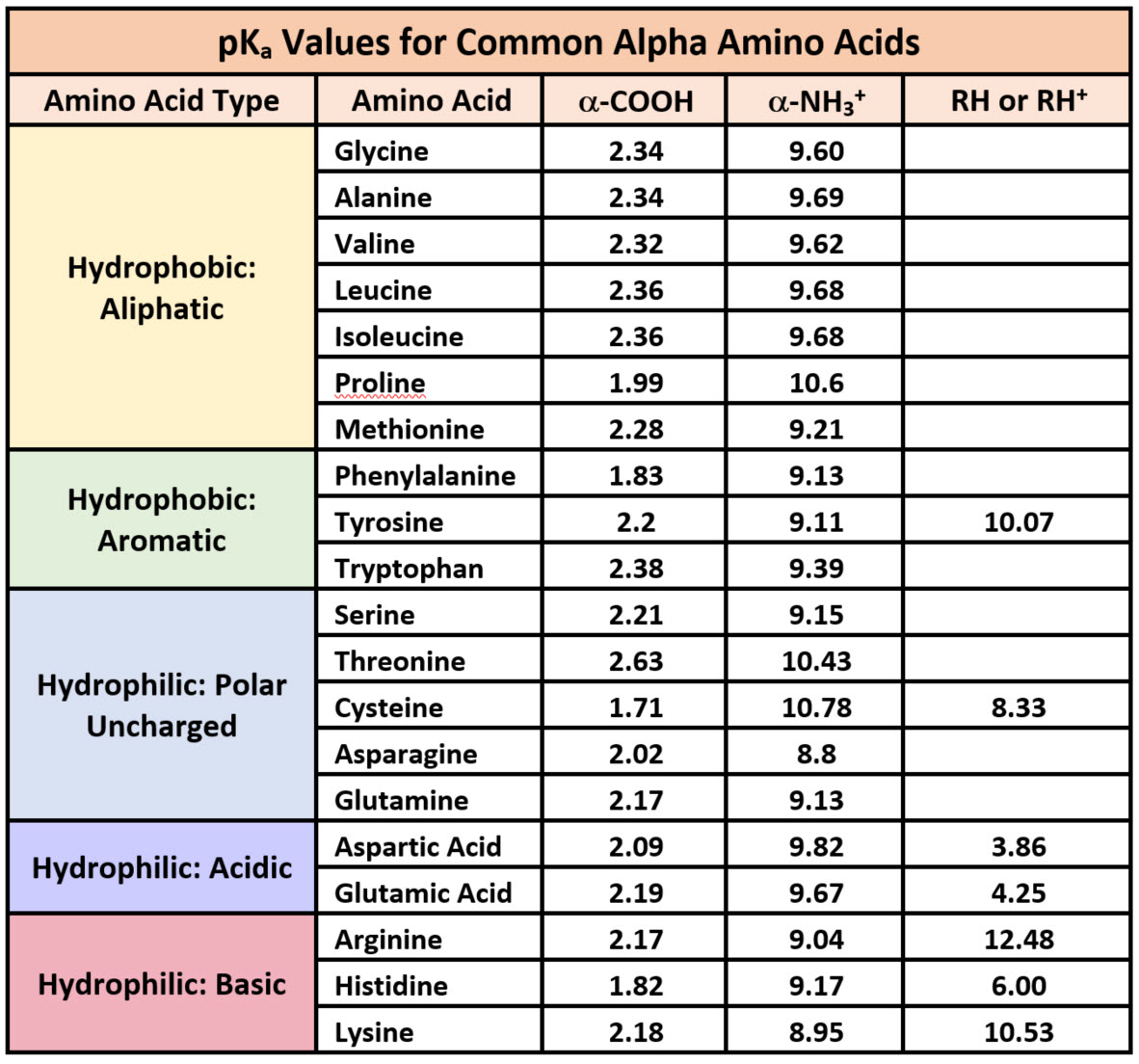
In addition, many hydrophobic amino acids are exposed on the surface and it is these that give a native protein its degree of hydrophobicity. Hydrophobic interactions stabilize the tertiary and quaternary structure of proteins. Certain amino acids are hydrophobic in order of decreasing hydrophobicity, they are tryptophan, norleucine, phenylalanine, tyrosine, leucine, valine, methionine, and alanine. Amino acids can be classified as acidic, basic, polar uncharged, or non-polar (hydrophobic). The degree of hydrophobicity of a protein is dependent on its amino acid sequence. The solubility of a peptide is primarily dependent on the physical properties of its amino acids. Less hydrophobic amino acids (glycine and L-alanine) disrupt hydrogen bonds between water molecules to inhibit hydrate formation while more hydrophobic amino acids (L-valine, L-leucine and L.

It is the structure of the water that creates hydrophobic interactions. The energetics of hydrophobic bond formation drives amino acids with hydrophobic sidechains into the interior of proteins, in most cases this is a very. Hydrophobic interactions are forced on nonpolar compounds by the polar environment.


 0 kommentar(er)
0 kommentar(er)
